delta g for spontaneous reaction|delta g is equal to : Clark Gibbs free energy (G) is a state function defined with regard to system quantities .
WEBParis vs Bayern: facts. Paris vs Bayern 2020/21. All UEFA Champions League match information including stats, goals, results, history, and more.
0 · spontaneity chart chemistry
1 · is positive delta g spontaneous
2 · if enthalpy is negative spontaneous
3 · delta g spontaneous chart
4 · delta g q problems
5 · delta g is positive
6 · delta g is equal to
7 · delta g chemistry chart
8 · More
Viego is a champion in League of Legends. This article sectio.
delta g for spontaneous reaction*******The sign of ΔG indicates the direction of a chemical reaction and determine if a reaction is spontaneous or not. \( \Delta G < 0 \): reaction is spontaneous in the direction written (i.e., the reaciton is exergonic) \( \Delta G =0 \): the system is at .Quantum correction; Contributors and Attributions; Helmholtz energy function .
Therefore according to the second equation, the \(\Delta{H}\) will also be .
No headers. Entropy is a state function that is often erroneously referred to as the .A spontaneous reaction is one that releases free energy, and so the sign of \(\Delta .Gibbs free energy (G) is a state function defined with regard to system quantities .
Learn how to use the second law of thermodynamics and Gibbs free energy to predict whether a reaction is spontaneous, endergonic, or at equilibrium. See ex.A spontaneous reaction is one that releases free energy, and so the sign of \(\Delta G\) must be negative. Since both \(\Delta H\) and \(\Delta S\) .
Gibbs free energy (G) is a state function defined with regard to system quantities only and may be used to predict the spontaneity of a process. A negative .
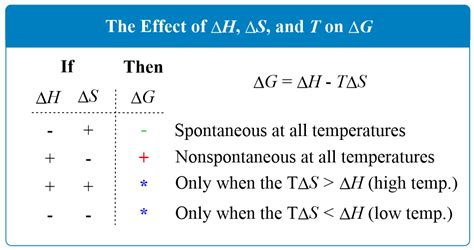
delta g is equal to So in this sense a negative value of ΔG° still means a spontaneous reaction, but the numerical value represents the theoretical maximum amount of energy free to do work. . So, when we do the math, delta G naught for this reaction is equal to .delta g for spontaneous reaction For example, when Mr. Khan was talking about a spontaneous Gibbs free energy process - if delta H (enthalpy) was positive and delta S (entropy) was positive, the temperature would need to have a "High T" or high enough to make delta G (Gibbs .So if you had to calculate the Gibbs free energy change at, say, 298 K, you can just slot the numbers in: ΔG° = ΔH° - TΔS°. ΔG° = -890.4 - 298 (-0.2442) = -817.6 kJ mol -1. It is .
The change in Gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the spontaneity of a reaction. Free-energy .The change in Gibbs free energy (ΔG) for a system depends upon the change in enthalpy (ΔH) and the change in entropy (ΔS) according to the following equation: ΔG = ΔH - TΔS. ΔGo = ΔHo - TΔSo. The relationship holds true under standard conditions or .below this temperature the reaction is spontaneous. 2) Determine the Delta G under standard conditions using Gibbs Free Energies of Formation found in a suitable Thermodynamics table for the following reaction: . Gibbs free energy (G) is a state function defined with regard to system quantities only and may be used to predict the spontaneity of a process. A negative value for ΔG indicates a spontaneous process; a positive ΔG indicates a nonspontaneous process; and a ΔG of zero indicates that the system is at equilibrium. A number of .
For spontaneous reaction, ΔG is: Which of the following is not correct? (a) Δ G is zero for a reversible reaction. (b) Δ G is positive for a spontaneous reaction. (c) Δ G is negative for a spontaneous reaction. (d) Δ G is positive for a non-spontaneous reaction. Assertion :There is no reaction known for which ΔG is positive, yet it is .
Each line crosses from one spontaneity domain (positive or negative Δ G) to the other at a temperature that is characteristic of the process in question. This temperature is represented by the x -intercept of the line, that is, the value of T for which Δ G is zero: ΔG = 0 = ΔH − TΔS (19.7.4) (19.7.4) Δ G = 0 = Δ H − T Δ S. Q is our reaction quotient; It tells us where we are in the reaction, and remember, it has the same form as the equilibrium constant K. Delta G zero is the standard change in free energy, so the change in free energy under standard conditions. R is the gas .
delta g for spontaneous reaction delta g is equal tobelow this temperature the reaction is spontaneous. 2) Determine the Delta G under standard conditions using Gibbs Free Energies of Formation found in a suitable Thermodynamics table for the following reaction: 4HCN(l) + 5O 2 (g) ---> 2H 2 O(g) + 4CO 2 (g) + 2N 2 (g). Check to make sure the equation is balanced ; Look up the Standard . The change in Gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the spontaneity of a reaction. Free-energy change: ΔG = ΔH − TΔS\nonumber. Standard free-energy change: ΔG° = ΔH° − TΔS°\nonumber. We can predict whether a reaction will occur spontaneously by combining the entropy . The free energy change of a reaction is a mathematical combination of the enthalpy change and the entropy change. ΔGo = ΔHo − TΔSo (11.5.3) (11.5.3) Δ G o = Δ H o − T Δ S o. The symbol for free energy is G G, in honor of American scientist Josiah Gibbs (1839 - 1903), who made many contributions to thermodynamics.Example 18.6.1. In Example 18.5.3, we calculated that ΔG° = −32.7 kJ/mol of N 2 for the reaction N 2 (g)+3H 2 (g ⇌ 2NH 3 (g) This calculation was for the reaction under standard conditions—that is, with all gases present at a partial pressure of 1 atm and a temperature of 25°C. Calculate ΔG for the same reaction under the following nonstandard .

To determine if a reaction is spontaneous, use this formula to find Delta G. Gibbs Free Energy is NEGATIVE for spontaneous reactions.You can also determine .So, yes negative ∆ G means a spontaneous reaction. Suggest Corrections. 2. Q. Q. Q. Why is the slope negative what does it mean in a reaction. Q. Assertion :An electrochemical cell can be set-up only if the redox reaction is spontaneous. Reason: A reaction is spontaneous if free energy change is negative.Have a look at the Gibbs free energy formula (where d denotes delta): dG = dH - T*dS. as long as G remains negative, the reaction will be spontaneous. If H is positive, and S is positive, then the T*dS term must simply be larger than dH to .We have identified three criteria for whether a given reaction will occur spontaneously (that is, proceed in the forward direction, as written, to reach equilibrium): ΔS univ > 0, ΔG sys < 0, and the relative magnitude of the reaction quotient Q versus the equilibrium constant K. Recall that if K > Q, then the reaction proceeds spontaneously to the right as written, .
webAssista nossos canais de TV online 24 horas, com qualidade de transmissão superior e sem travamentos. Desfrute de uma experiência imersiva e flexível em qualquer dispositivo, a .
delta g for spontaneous reaction|delta g is equal to